



Juvéderm® Vollure XC
Juvéderm® Vollure XC
Couldn't load pickup availability
DESCRIPTION
DESCRIPTION
*Online purchase is only available to current patients of our office
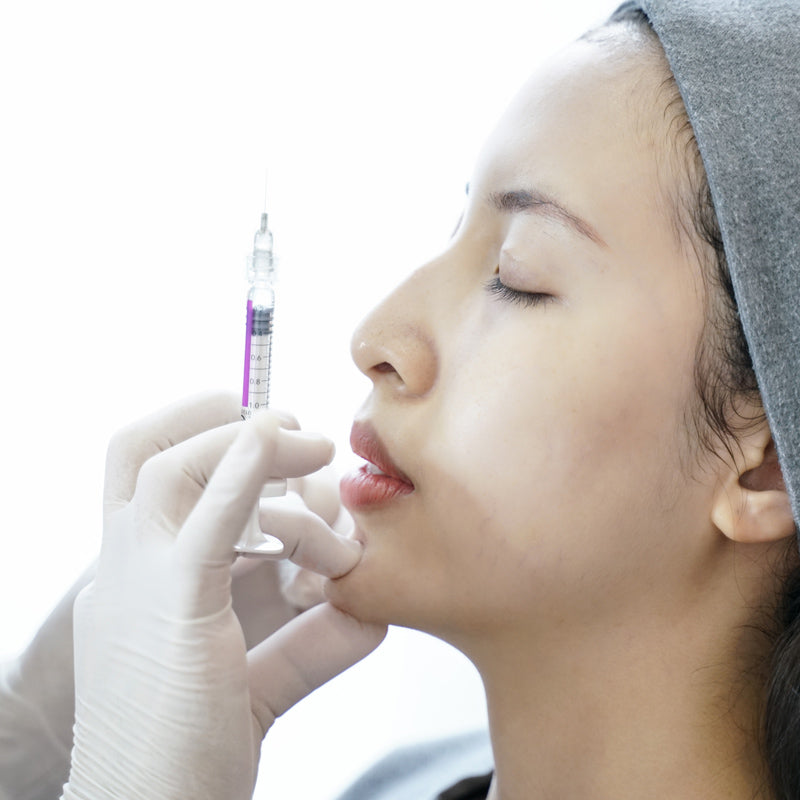
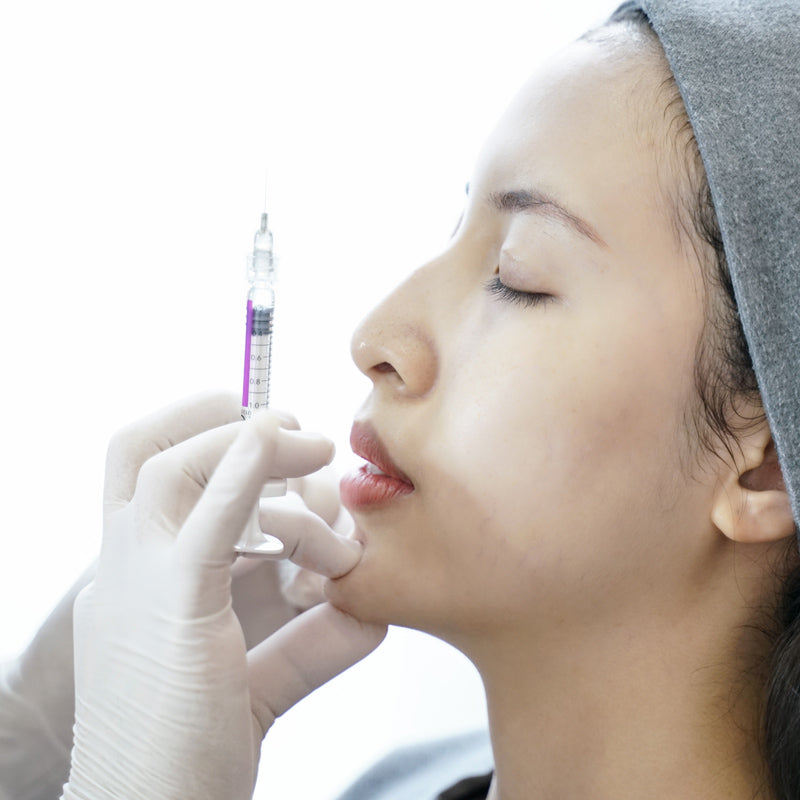
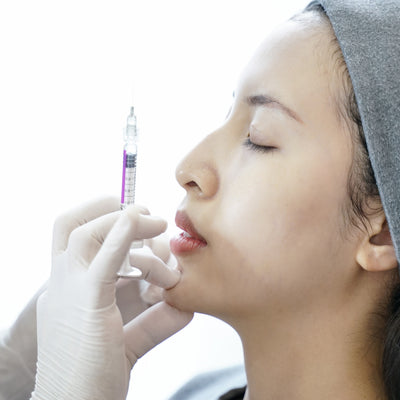
JUVÉDERM® VOLLURE™ XC is an FDA-approved injectable gel formulated to correct moderate to severe facial wrinkles and folds, such as nasolabial folds (smile lines) and marionette lines, in adults over 21.
Powered by VYCROSS® technology, this advanced filler blends different molecular weights of hyaluronic acid to create a smooth, long-lasting formulation that balances gel firmness with flexibility. This ensures natural-looking results that move with your expressions while maintaining volume where needed.
Clinical studies show that results can last up to 18 months with optimal treatment, making it one of the most durable fillers for wrinkle correction.
Benefits
- Softens nasolabial folds and marionette lines
- Smooths facial wrinkles for a youthful look
- Natural feel and appearance
- Results last up to 18 months
FAQs
FAQs
USAGE
USAGE